Corrosion Resistance of Smileloc Exposed to the Oral Environment
Contents
Executive Summary
The RODO abutment system has been designed to protect the Smileloc sleeve from contact with body fluids. In this study, the corrosion resistance of the abutment system under worst-case conditions where the coping becomes disengaged and the Smilelocs are exposed to an electrolyte solution is evaluated. Galvanic, pitting and crevice corrosion testing showed that the corrosion resistance of the RODO abutment system is greater than orthodontic archwires, which are regularly exposed to saliva, even under abnormal circumstances. Additionally, the amount of nickel leaching from the Smileloc sleeves due to corrosion is extremely low when compared to the consumption of nickel by typical US adults. Therefore, it is concluded that the RODO device has appropriate corrosion resistance for its indication for use.
Introduction
The harsh oral environment significantly affects the durability and performance of metallic dental devices. Because of patients’ dietary habits and oral hygiene, these devices are subjected to constantly changing conditions, such as varying pH, temperature, and saliva composition.1 This complex environment can promote corrosion, which can manifest in many different forms. For instance, crevice corrosion can occur whenever moisture is trapped in a narrow region between two surfaces, such as implantabutment screw joint interfaces. Additionally, pitting corrosion can occur on otherwise resistant surfaces in the presence of chloride and sulphide ions, which can cause protective oxide films to locally break down and the underlying metal to subsequently corrode. These pits can act as stress concentrators that promote fatigue failure when located in a critical load path. Galvanic corrosion occurs when two dissimilar metals and alloys are in electrical contact in an aqueous environment. Regardless of the mechanism, corrosion can compromise a device’s mechanical integrity and biocompatibility.1-3 As a metallic dental device, the RODO abutment system is susceptible to corrosion under the right conditions. This includes pitting and crevice corrosion, which could occur in the narrow regions between the Smileloc sleeve and the implant abutment and coping surfacCorrosion Resistance of Smileloc Exposed to the Oral Environment Author: Chase S. Linsley, PhD EXECUTIVE SUMMARY The RODO abutment system has been designed to protect the Smileloc sleeve from contact with body fluids. In this study, the corrosion resistance of the abutment system under worst-case conditions where the coping becomes disengaged and the Smilelocs are exposed to an electrolyte solution is evaluated. Galvanic, pitting and crevice corrosion testing showed that the corrosion resistance of the RODO abutment system is greater than orthodontic archwires, which are regularly exposed to saliva, even under abnormal circumstances. Additionally, the amount of nickel leaching from the Smileloc sleeves due to corrosion is extremely low when compared to the consumption of nickel by typical US adults. Therefore, it is concluded that the RODO device has appropriate corrosion resistance for its indication for use. www.rodomedical.com | Page 2 RODO UNIVERSITY es, as well as galvanic corrosion, which could occur at the interfacing surfaces of the nitinol sleeve and the titanium abutments and copings. However, a seal between the implant abutment cuff and the restoration protects the Smileloc from contact with any material, including saliva, and in the absence of an electrolyte solution, corrosion does not occur. In the worst-case scenario where there is a leaky abutment seal, the conditions necessary for corrosion to occur exist. Therefore, the purpose of these studies was to evaluate the electrochemical behavior of the RODO abutment system’s metal components in an electrolyte solution to ensure safe use even in worst-case conditions
Background
Corrosion is a reduction-oxidation reaction that involves simultaneous charge and mass transfer across a metal-solution interface. There are four conditions necessary for corrosion to occur: 1) an anodic reaction; 2) a cathodic reaction; 3) a metallic path of contact between anodic and cathodic sites; and 4) the presence of an electrolyte.4 For most metals, oxidation is thermodynamically spontaneous under ambient conditions. The reactions can be split into two half-cell reactions, which are defined as a reaction where electrons appear on either the product or reactant side of a reaction. The anodic reaction is the oxidation reaction:
M → Mn+ + ne-
In this half of the reaction, n number of free electrons are produced by the reaction and a loss of metal, M, occurs. The cathodic reaction is the reduction reaction since electrons are consumed. There are several possible cathodic reactions, which are dependent on the pH of the electrolyte solution and the chemical species present (e.g. dissolved oxygen):
2H+ + 2e- → H2 (acidic)
O2 + 4H+ + 4e- → 2H2O
O2 + 2H2O + 4e- → 4OH- (neutral/basic)
These anodic and cathodic reactions occur in a coupled manner at different places on the metal surface or, in the case of galvanic corrosion, on the surfaces of dissimilar metals in electrical contact with one another.
The metallic atoms most likely to pass into solution are the ones located at the highest energy sites. High-energy sites on metal surfaces exist where the crystalline environment is less stable. These locations are at defects in the crystal structure, such as grain boundaries, edges, steps, kink sites, screw dislocations and point defects.4 Surface contaminants, such as impurity metal atoms or the adsorption of ions from solution, can also change the surface energy of the underlying metal atoms. It is because of the heterogeneity of metal surfaces that anodic and cathodic reactions can occur on the same surface. During galvanic corrosion, the more stable metal acts as the cathode and the more chemically active metal acts as the anode. There are several factors that affect the rate of galvanic corrosion, including the potential difference and physical distance between the two metals, the surface area of the anode and cathode, as well as the corrosiveness of the surrounding media.4
Materials and Methods
Surface area is a factor that can affect corrosion performance. For instance, a large surface area can result in lower breakdown potential because more sites are susceptible to pitting and crevice attacks. Smilelocs designed for posterior jaw applications have the largest surface area. Therefore, they are considered the worst-case scenario and were used for this study. In galvanic corrosion testing, the surface area ratio of anode to cathode affects the rate of galvanic corrosion – a galvanic couple with a smaller anode to cathode surface area ratio results in higher corrosion rates. In the RODO abutment system, the nitinol Smileloc is the anode, while the titanium abutment, coping, and implant are the cathode. Therefore, Smileloc and corresponding abutment, coping and implant with the smallest nitinol to titanium surface area ratio will result in higher rates of corrosion and were used to study galvanic corrosion.
To prepare the samples for the pitting and crevice corrosion study, conducted per ASTM F2129,5 the nitinol Smileloc sleeve and the three titanium components (implant, abutment and coping) were assembled but the coping remained disengaged so as to leave the Smileloc exposed to the test solution (worst-case condition). The surface area of the Smilelocs being tested was 1 cm2.
To prepare the samples for the galvanic corrosion study, conducted per ASTM G71,6 the three titanium components (implant, abutment and coping), with a combined surface rea of 3-5 cm2, were assembled so that they could be tested in combination against the nitinol Smileloc sleeve, which had a surface area of 1 cm2. The nitinol Smileloc is the anode, and the titanium components were the cathode.
A firm joint between the sample and a stainless steel wire lead was formed using a conductive epoxy. The joint and the submerged section of stainless steel wire lead were insulated using three layers of nonconductive lacquer, which was applied sequentially and allowed to dry for at least 8 hours.
| Sample | Open Circuit Potential | Breakdown Potential | ||||
|---|---|---|---|---|---|---|
| Min (Vse) | Max (Vse) | Avg (Vse) | Min (Vse) | Max (Vse) | Avg (Vse) | |
| Orthodontic Archwire | 0.259 | -0.187 | -0.213 | 0.156 | 0.284 | 0.230 |
| RODO Device with Control Smileloc | -0.246 | -0.160 | -0.206 | 0.774 | No Breakdown (1.00) | 0.895 |
| RODO Device with Clinical Smileloc used for 6 mo. | -0.206 | -0.059 | -0.119 | 0.922* | No Breakdown (1.00) | 0.987 |
| *Only one sample exhibited breakdown on reverse polarization scan. | ||||||
All glassware and corrosion cells were cleaned and rinsed with deionized (DI) water. The test cell was filled with 0.9% NaCl phosphate buffered saline (PBS) test solution, and the salt bridge was filled with the same test solution. No visible air bubbles were present. The test cell was submerged in a 37°C (± 1°C) water bath. When the temperature of the solution stabilized, dissolved oxygen was removed from the test solution by flushing with nitrogen gas at a flow rate of 150 cm3/min for at least 30 min, which continued for the duration of the test. Prior to starting the test, the temperature and pH value of the test solution was recorded. The sample was immersed and the tip of the salt bridge was adjusted to be 2 mm away from the sample surface. The open-circuit potential was recorded for 60 min followed by a cyclic potentiodynamic polarization scan at a scan rate of 1 mV/sec. The scan was reversed when breakdown was observed (i.e. current density reached threshold of 2 decades), or if no breakdown was observed, at 1000 mV. The pH of the test solution was measured at the conclusion of the test. Samples that underwent galvanic corrosion testing had the current and potential measured every 60 seconds for at least 24 hours. Any irregularities during the testing, as well as discoloration or degradation of the test solution were noted.
Results and Discussion
Pitting, crevice and galvanic corrosion of the RODO abutment system were studied. In normal use, the abutment seal protects the Smileloc from contact with any material including body fluids. The purpose of this study was to evaluate the corrosion resistance of the abutment system under worst-case conditions where the coping becomes disengaged and the Smilelocs are exposed to an electrolyte solution. The corrosion resistance was compared against nitinol orthodontic archwire (control).
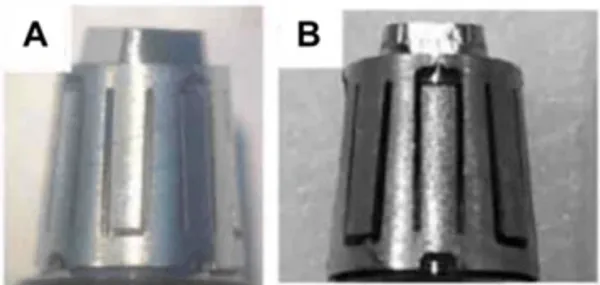
Both unused Smilelocs and Smilelocs that had been used for 6 months clinically did not display any visible signs of major corrosion after pitting and crevice corrosion testing (Fig. 1). Compared to orthodontic archwires, which are also used in the dental cavity and regularly exposed to saliva, both new and used RODO devices in a worst-case scenario condition have higher breakdown potentials (Table 1). Breakdown potentials for orthodontic archwires ranged from 0.156 to 0.284 VSE. In contrast, all test RODO devices had much higher breakdown potentials, ranging from 0.774 VSE to no breakdown for unused samples and from 0.922 VSE to no breakdown potential for RODO devices retrieved after 6 months use in clinical trial patients. Fig. 2 shows polarization curves for orthodontic archwires and RODO devices in 0.9% NaCl PBS test solution at 37°C. In addition to localized loss of thickness, corrosion pits can be harmful by acting as stress risers. Cracks caused by cyclical stresses may initiate at the base of corrosion pits, which can be enough to produce catastrophic failure of the device.7
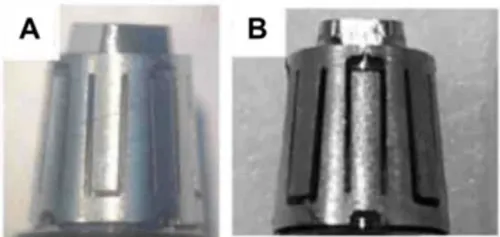 Figure 1. Representative macrographs of (A) control, and (B) clinical Smilelocs after potentiodynamic polarization.
Figure 1. Representative macrographs of (A) control, and (B) clinical Smilelocs after potentiodynamic polarization. The RODO abutment system’s design includes contact between a nitinol sleeve with a set of arms that engage a titanium abutment and another set of arms that engage a titanium coping. By using nitinol, shape memory can be used to rapidly unlock dental prostheses from implant abutments making retrievability and replacement a much simpler process. However, contact between dissimilar metals can lead to galvanic corrosion in an electrolyte solution – conditions that exist in the oral environment. Although there is no specified minimum duration for galvanic testing per ASTM G71, it is recommended by the new standard ASTM F3044-2014 that galvanic tests have a minimum duration of 24 hours.8 As it can be appreciated from Fig. 3, the RODO devices exhibited reasonably stable current and potential after 24 hours. These tests indicate that the combination of nitinol with titanium is stable, with the potential becoming less active over time. Furthermore, the corrosion current decreased with time.
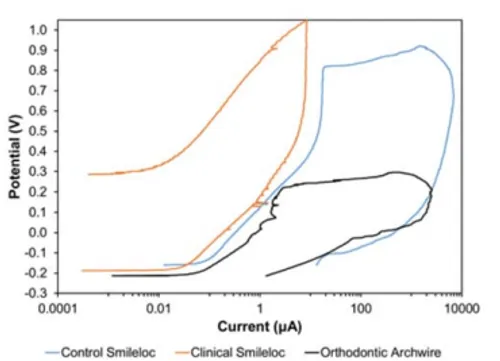 Figure 2. Representative polarization curves of orthodontic archwire, control Smilelocs and clinical Smilelocs in 0.9% NaCl PBS test solution at 37°C.
Figure 2. Representative polarization curves of orthodontic archwire, control Smilelocs and clinical Smilelocs in 0.9% NaCl PBS test solution at 37°C. Because corrosion involves the simultaneous transfer of charge and mass across the metal/solution interface, corrosion rates can be calculated from electrochemical testing. The Faraday (F) is the link between charge transfer and mass transfer. Faraday’s First Law of Electrolysis states that the mass (m) of a corroded metal is directly proportional to the amount of charge passed through the solution of an electrolyte. It can be summarized by
m = QM/Fz
where Q is the total charge passed through the metal in coulombs, M is the molar mass of the metal in grams per mol, and z is the number of equivalents transferred per mole of metal.
Corrosion rate and mass loss rate were calculated per ASTM G102 using the final 15 minutes of the test.9 As the current was generally steady or decreasing, the calculated rates represent upper limits to the true value of galvanic corrosion rates over and extended implantation period. The calculated corrosion and mass loss rates were very low, with values of 5x10-6 – 1x10-5 mm per year and 0.01-0.02 μg/day. Based on the stoichiometry of nitinol, only 56% of this mass loss (or approximately 0.006-0.011 μg/day) would be nickel. This is extremely low when compared to the consumption of nickel by typical US adults, which ranges between 69 and 162 μg/day,10 which is several orders of magnitude larger than those measured for the RODO device. Additionally, the corrosion mass loss rate of the nitinol device was also far lower than nickel leaching data from commercially available orthodontic nitinol archwire, which is approximately 13.05 μg/day.11 These results are corroborated by nickel leaching testing conducted on pairs of RODO abutment samples submerged in 0.9% NaCl PBS solution at 37°C (± 1°C), with a surface area to volume ratio of 1 cm2/mL. Note that these samples were also tested with Smileloc exposed to PBS solution, which is considered worstcase condition since the abutment seal protects the Smileloc from contact with any material, including saliva, during normal use. The results showed that nickel release was greatest the first week of submersion (up to 0.12 μg/day) and decreased to as little as 0.026 μg/day for the remaining three weeks of the test. The concentration of nickel released was measured by inductively coupled plasma mass spectrometry (ICP-MS), which has a detection limit of 10 ppb.
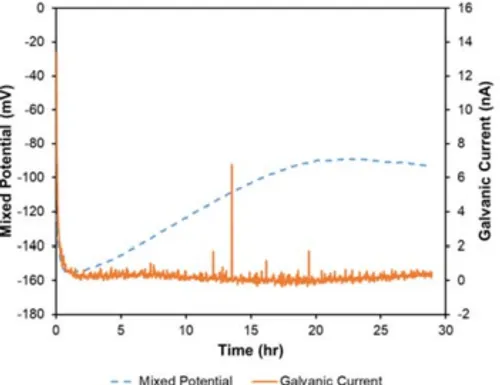 Figure 3. Representative galvanic current and mixed potential
curves for the RODO abutment system – titanium implant, abutment and coping, and the nitinol Smileloc sleeve – over 29 hr.
test period in 0.9% NaCl PBS test solution at 37°C. The nitinol
Smileloc sleeve is the anode, and the titanium components were
the cathode.
Figure 3. Representative galvanic current and mixed potential
curves for the RODO abutment system – titanium implant, abutment and coping, and the nitinol Smileloc sleeve – over 29 hr.
test period in 0.9% NaCl PBS test solution at 37°C. The nitinol
Smileloc sleeve is the anode, and the titanium components were
the cathode.