Assessment of Nickel Ion Release from Smileloc Sleeves

Contents
Executive Summary
Nickel is an allergen and the exposure risk from medical and dental devices made of alloys containing nickel requires thorough investigation. The amount of nickel that leached from the Smileloc sleeves, which were fully submerged in 0.9% NaCl PBS solution (simulating worst-case scenario where the Smileloc sleeve becomes disengaged from the coping and is exposed to the oral environment), was greatest during the first week of submersion – up to 0.12 μg/day – and decreased to at most 0.026 μg/day for the remaining three weeks of the test. These values are orders of magnitude lower than the average dietary intake of nickel for adults globally, which has been reported to be between 100 to 300 μg/day. These results, combined with the electrochemical testing previously described, show that nickel leaching from the Smileloc sleeve is insignificant and therefore safe for its indication for use.
Introduction
Nickel is the most common cause of metal allergy with Centers for Disease Control and Prevention (CDC) estimating that between 10-20% of the general population is sensitized to nickel.1 Most metal allergies develop due to environmental exposure, and nickel can be found in several everyday objects, such as coins, jewelry, dental materials, and mobile phones. Oral exposure appears to cause allergic reactions in a dose-dependent fashion in nickelsensitive individuals; however only the most sensitive individuals react to nickel levels in daily diet.2 Clearly, a thorough understanding of the nickel release rates from commercially available products is required to not only protect those users with existing nickel allergies, but is also required to protect users from becoming sensitized.
The Smileloc sleeve uses nitinol – a nickel-titanium alloy that undergoes martensitic transformation to switch between a locked and unlocked configuration. Nickel release from nitinol is significantly decreased by the formation of a thermodynamically stable titanium oxide passivation layer, but because nickel containing alloys used in medical and dental devices are exposed to the harsh physiological environment, some nickel leaching is likely to occur during use. Assessment of Nickel Ion Release from Smileloc Sleeves Author: Chase S. Linsley, PhD EXECUTIVE SUMMARY Nickel is an allergen and the exposure risk from medical and dental devices made of alloys containing nickel requires thorough investigation. The amount of nickel that leached from the Smileloc sleeves, which were fully submerged in 0.9% NaCl PBS solution (simulating worst-case scenario where the Smileloc sleeve becomes disengaged from the coping and is exposed to the oral environment), was greatest during the first week of submersion – up to 0.12 μg/day – and decreased to at most 0.026 μg/day for the remaining three weeks of the test. These values are orders of magnitude lower than the average dietary intake of nickel for adults globally, which has been reported to be between 100 to 300 μg/day. These results, combined with the electrochemical testing previously described, show that nickel leaching from the Smileloc sleeve is insignificant and therefore safe for its indication for use. www.rodomedical.com | Page 2 RODO UNIVERSITY Therefore, the goal of this study was to investigate the amount of nickel that leaches from the Smileloc sleeve under the worst-case scenario where the Smileloc sleeve becomes disengaged from the coping and the RODO device is exposed to the oral environment for one month. During normal use, the abutment seal protects the Smileloc sleeve from contact with any material, including body fluids
Materials and Methods
The materials used in this study are summarized in Table 1 and each assembled device included a commercially available titanium implant, a compatible RODO abutment also made of titanium, a nitinol Smileloc sleeve, and a titanium coping. A total of 8 assembled RODO devices in finished and sterilized condition were tested. Because larger surface areas exposed to the test medium can leach higher amounts of nickel, the Smileloc sleeves with the largest surface area (F-Smileloc, 1.1 cm2 ) were used in this study.
| Implant | RODO Abutment | |||
|---|---|---|---|---|
| Set 1 | Straumann BL 3.3 mm x 14 mm SLA | Straumann BL NC 1.0 mm | ||
| Straumann BL 3.3 mm x 14 mm SLA | Straumann BL NC 1.0 mm | |||
| Set 2 | Straumann BL 3.3 mm x 14 mm SLA | Straumann BL NC 1.0 mm | Smileloc | Milled Ti Coping |
| Astratech Osseospeed TX 3.5S x 13 mm | Astratech Aqua 0.5 mm | |||
| Set 3 | Straumann BL 4.8 mm x 10 mm SLA | Straumann BL RC 2.5 mm | ||
| Nobel Replace WP 5.0 mm | Nobel Replace WP 2.0 mm | |||
| Set 4 | Straumann BL 4.8 mm x 10 mm SLA | Straumann BL RC 0.5 mm | ||
| Straumann BL 3.3 mm x 14 mm | Straumann BL NC 1.0 mm | |||
| BL = Bone Level; NC = Narrow CrossFit®; RC = Regular CrossFit®; Ti = Titanium; WP = Wide Platform | ||||
Phosphate buffered saline (PBS) with 0.9% NaCl was used as the test medium, and a nitinol surface area to test medium volume ratio of 1 cm2 /mL was used in this study. Due to the low volume of solution needed to test each device, the devices were tested in pairs so solution volumes large enough to completely submerge the devices could be used. Briefly, each set (consisting of 2 RODO devices) was submerged in 2 mL of 0.9% NaCl PBS solution in glass vials. The vial was placed in a circulating water bath set at 37°C (±1°C). At each time point, the vials were ultrasonically agitated 5 min to uniformly distribute leached nickel ions throughout the test medium. The test medium was collected and replaced with fresh 0.9% NaCl PBS solution to maintain sink conditions. Prior to adding fresh test medium, the test devices were dried by shaking and blow-drying. This process was repeated every 24 hr for the first 7 days and then every 3 to 4 days until day 30. Inductively coupled plasma mass spectrometer (ICP-MS) was employed to measure the concentration of nickel ions that had leached from the Smileloc sleeve. The analytical detection limits for nickel was 10 ppb. As a reference, the nickel concentration of 2 samples of fresh 0.9% NaCl PBS test medium were measured prior to the test solutions
Results and Discussion
This study was designed to simulate the worst-case scenario where the Smileloc sleeve becomes disengaged from the coping and the RODO device is exposed to the oral environment. During normal use, the abutment seal protects the Smileloc sleeve from contact with any material, including body fluids, thereby protecting it from corrosive processes. To validate the test setup and recovery method, a spike and recovery test was performed. Briefly, four glass vials filled with the same batch of 0.9% NaCl PBS solution used for the test samples were spiked with 100 ppb of nickel. Two of these vials were immediately analyzed for nickel concentration and the remaining two were aged for 15 days at 37°C in the same vials used for the submerging the RODO devices. Ninety-seven and 100 ppb nickel was recovered from the two spiked samples that were immediately analyzed and 90 ppb nickel was recovered from the spiked and aged samples. These results showed the test method and materials, including vials, were appropriate to accurately measure nickel release by the test devices.
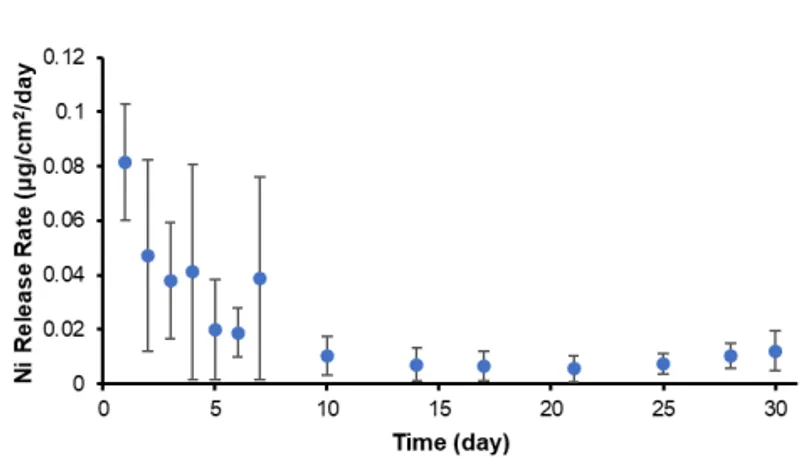 Fig. 1. Nickel release rates from asreceived RODO devices fully submerged in
0.9% NaCl PBS solution for 30 days (n=8).
The release rates were calculated by dividing the accumulated amounts of nickel
released between subsequent sampling
periods by the exposure time between
sampling periods. The greatest nickel release rate – 0.109 μg/cm2
/day – was measured after the first day of immersion, which
decreased to as much as 0.024 μg/cm2
/day
for the final three weeks of the test.
Fig. 1. Nickel release rates from asreceived RODO devices fully submerged in
0.9% NaCl PBS solution for 30 days (n=8).
The release rates were calculated by dividing the accumulated amounts of nickel
released between subsequent sampling
periods by the exposure time between
sampling periods. The greatest nickel release rate – 0.109 μg/cm2
/day – was measured after the first day of immersion, which
decreased to as much as 0.024 μg/cm2
/day
for the final three weeks of the test. The results from fully immersed RODO devices showed that nickel release was greatest the first week of submersion in 0.9% NaCl PBS solution – up to 0.12 μg/day – and decreased to as much as 0.026 μg/day for the remaining three weeks of the test (Fig. 1). A similar release profile has been observed in previous studies measuring nickel release from nitinol in simulated biological fluids.3,4 The initial burst release of nickel ions from nitinol is likely determined by the concentration of nickel in the outer-most surface layers, which can be influenced by surface preparation method.5,6 The release rate of nickel ions from the Smileloc sleeve is orders of magnitude less when compared to nickel leaching from orthodontic devices, such as nitinol archwires and stainless steel brackets. For instance, the average nickel release rate for a single arch of stainless steel brackets banded with nitinol archwires submerged in simulated saliva was 13.05 μg/day.7 A study investigating nickel release from several commercially available nitinol archwires (wire length= 28 cm), with and without mechanical loading, found comparable range of release rates: 1-8 μg/day.8 In all cases, these values are orders of magnitude lower than the average dietary intake of nickel for adults globally, which has been reported to be between 100 to 300 μg/day, depending on dietary habits.9 Protection from the oral environment provided by the RODO abutment seal will further limit the amount of nickel that leaches from the Smileloc sleeve.
 Fig. 2. RODO devices show no signs of corrosion after 30 days of being fully immersed in 0.9% NaCl PBS solution.
Fig. 2. RODO devices show no signs of corrosion after 30 days of being fully immersed in 0.9% NaCl PBS solution. Mass loss rates calculated from electrochemical testing that was conducted following ASTM G102 estimated slightly lower nickel ion release rates, but nonetheless consistent with the nickel leaching test results. Again, the worst-case scenario where the Smileloc sleeve was continuously exposed to saliva was investigated. The calculated mass loss rates were 0.01-0.02 μg/day, and based on the stoichiometry of nitinol, only 56% of this mass loss (or approximately 0.006-0.011 μg/day) would be nickel. Electrochemical techniques often underestimate the corrosion rates predicted by other techniques, such as weight loss and quantity of dissolved ions measurements,10 because they only capture the corrosion behavior of a specimen during the narrow window of time over which the measurement was carried out. However, visual inspection of the devices under 20X magnification following 30 days of immersion in 0.9% NaCl PBS solution shows no signs of corrosion (Fig. 2) – further supporting the low corrosion risk and subsequent exposure to nickel.