Accuracy and Tolerance of RODO Abutment Critical Dimensions
Contents
Executive Summary
Geometric and dimensional analysis was used to establish the dimensional tolerances for RODO Medical’s abutment interfaces for Nobel, Neodent and Straumann implants. This ensures compatibility, fit, and performance equivalent to the FDA-cleared abutments for each of the implant brands. Fatigue testing has validated these tolerances and they have been cleared by the FDA. These high-quality components make the dental treatment outcomes more predictable with fewer failure incidents.
Introduction
Dimensional analysis ensures RODO Medical’s precision machined abutment is compatible with most commercial dental implant fixtures. These abutments accept Smileloc – a shape memory sleeve that has 2 sets of arms that switch between the “engaged” and “disengaged” positions that lock and unlock the restoration, respectively. Currently, RODO abutments are designed to fit with commercially available implant platforms. The presented geometric and dimensional analysis ensures the compatibility of RODO abutments with several of the topselling dental implant brands. .
Background
Reverse engineering techniques are employed to extract dimensional information for components that need to be duplicated or modified when computeraided design (CAD) models are unavailable or unusable [1]. This information is also used to design custom parts that interface with commercially available products for which the manufacturer is unable or unwilling to provide design specifications. An essential element of designing and manufacturing mechanical components is tolerancing. Tolerance can be defined as the permissible variation of a physical dimension from its nominal value and is important to determine for the proper assembly and performance of interfacing parts. Failure to consider tolerances of two interacting parts can negatively impact their stability and durability. This is especially true if the intent is to manufacture a commercial product from data collected using reverse engineering techniques. In these cases, an important goal of reverse engineering is to extract tolerance data from measurements, which is often done by performing dimensional anal- www.rodomedical.com | 2 RODO UNIVERSITY ysis on several specimens of the same product to elucidate normal manufacturing variations [2]. Reverse engineering was employed to design RODO abutments that precisely match commercially available dental implants. .
Materials and Methods
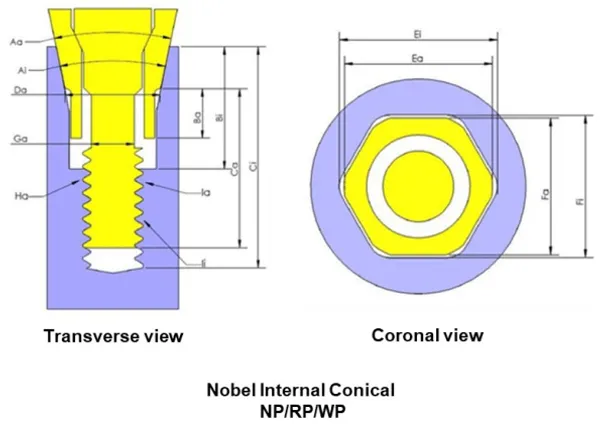
To design RODO abutments using reverse engineering, the following FDA-cleared implants and their approved abutments were used: Straumann’s Bone Level (BL) Narrow CrossFit (NC) and Regular CrossFit (RC) implants; Neodent’s Cone Morse (CM) implants; and Nobel’s Internal Conical 3.0, Internal Conical Narrow Platform (NP), Internal Conical Regular Platform (RP), and Internal Conical Wide Platform (WP), and Tri-Channel Narrow Platform (NP), Tri-Channel Regular Platform (RP), Tri-Channel Wide Platform (WP), and Tri-Channel 6.0 implants.
 Figure 1. Transverse and coronal views of FDA-cleared abutments
and abutment screws for Nobel implants
Figure 1. Transverse and coronal views of FDA-cleared abutments
and abutment screws for Nobel implants To design RODO abutments using reverse engineering, the following FDA-cleared implants and their approved abutments were used: Straumann’s Bone Level (BL) Narrow CrossFit (NC) and Regular CrossFit (RC) implants; Neodent’s Cone Morse (CM) implants; and Nobel’s Internal Conical 3.0, Internal Conical Narrow Platform (NP), Internal Conical Regular Platform (RP), and Internal Conical Wide Platform (WP), and Tri-Channel Narrow Platform (NP), Tri-Channel Regular Platform (RP), Tri-Channel Wide Platform (WP), and Tri-Channel 6.0 implants. an example. The CAD images are shown in contrasting colors such that the gaps between components of the system are displayed in white. Abutment features are noted with subscript “a”, while matching implant features are noted with subscript “i”. Some of the features deemed critical to fit and performance of the abutment-implant interface were: taper angle (Aa), engaging feature length across abutment flats and height (Fa and Ba, respectively). Taper angle and engaging feature height was measured on specimens using a digital optical metrological system (Micro-Vu Corp., Windsor, CA). The length across the abutment engaging flats was measured using a high-precision micrometer. For each feature, a minimum of 6 specimens from at least 2 different lots was measured and the average dimension, standard deviation, upper and lower tolerance limits were recorded along with the manufacturer’s information, part number, and lot number. A summary of the implants measured is shown in Table 1, and a summary of sample size for FDA-cleared abutments for each implant line is shown in Table 2.
RESULTS & DISCUSSION
To ensure proper fit, dimensions for the RODO abutment’s implant interface features were not allowed to impinge on the matching implant’s interface features, which could compromise the performance of the implant, the abutment, or the entire abutment-implant system. Additionally, the selected tolerances for critical features of the RODO abutment’s implant interface were all within ranges measured on FDA-cleared abutments (Table 3). Tolerances define realistic size and shape limits for assembled parts. The design requires that the components fit together tightly. Components that do not have adequate fit contribute to screw loosening, and abutment screw loosening is one of the most common causes of implant-supported restoration failure [3]. Therefore, it is critical that components have been designed to fit and work together long-term.
There are several design features that are critical to the performance of the RODO abutments, including the taper angle and engaging feature flats and height. The engaging feature flats on the RODO abutment (Feature Fa in Figure 1 coronal view) provide rotational resistance equivalent to that of FDAcleared abutments while not impinging on implant feature Fi . Anti-rotational features have previously been shown to significantly help maintain abutment screw preload and prevent loosening [4-6]. Loosening is important to minimize since motions between the abutment and the implant can cause wear and premature failure of the components. The presence of a taper has also been shown to have an effect on screw loosening since the tapered-interference fit helps to provide a secure connection between the abutment and the implant by the large contact pressure and resulting frictional resistance at the implant –abutment interface [7,8]. Additionally, the taper angle provides resistance to bending forces and increases the strength of the implant-abutment joint [9,10]. It should be noted that torque efficiency – the ratio between the loosening torque and the tightening torque, which is a measure of screw loosening prevention – and joint strength are competing design criteria since the fracture resistance of the implant collar is increased with greater taper angles while torque efficiency is decreased [7,9].
| Manufacturer | Implant Platform | n | lots |
|---|---|---|---|
| Neodent | Cone Morse | 6 | 6 |
| Nobel Biocare | NobelActive | 18 | 16 |
| Nobel Replace | 18 | 18 | |
| NobelSpeedy | 12 | 12 | |
| Straumann | Bone Level Narrow Crossfit | 6 | 6 |
| Bone Level Regular Crossfit | 6 | 6 |
| Manufacturer | Implant Platform | n | lots |
|---|---|---|---|
| Neodent | Cone Morse | 10 | 9 |
| Nobel Biocare | Internal Conical 3.0 | 6 | 2 |
| Internal Conical NP | 11 | 10 | |
| Internal Conical RP | 14 | 5 | |
| Internal Conical WP | 6 | 6 | |
| Tri-Channel NP | 6 | 5 | |
| Tri-Channel RP | 7 | 7 | |
| Tri-Channel WP | 8 | 8 | |
| Tri-Channel 6.0 | 9 | 4 | |
| Straumann | Bone Level Narrow Crossfit | 14 | 8 |
| Bone Level Regular Crossfit | 10 | 8 | |
| Abbreviations: Narrow Platform (NP); Regular Platform (RP); Wide Platform (WP). | |||
| Aa (°) | Ba (mm) | Fa (mm) | |
|---|---|---|---|
| Measured Min. | 29.6 | 2.05 | 2.31 |
| Measured Max. | 30.6 | 2.30 | 2.37 |
| Avg. Measured Value | 30.1 | 2.24 | 2.33 |
| Std. Deviation | 0.3 | 0.06 | 0.01 |
| RODO Min. | 29.9 | 2.08 | 2.33 |
| RODO Max. | 30.5 | 2.28 | 2.37 |
In addition to reducing micromovements that can lead to wear, proper fit between implants and abutments helps to reduce the size of microgaps that exist at their interface. Microgaps are susceptible to infiltration by oral fluids, glycoproteins, and microorganisms, which can further exacerbate disharmony between the two components. First, oral fluids can act as lubricants that reduce friction between the abutment and implant and enable micromovements [11]. Additionally, oral fluids and microorganisms can cause corrosion and loss of surface material comprising the contact area between the two components. Several studies have reported that the acidic metabolic byproducts of microorganisms have caused increased corrosion rates [12-14] as have therapeutic substances like bleaching agents and fluorides
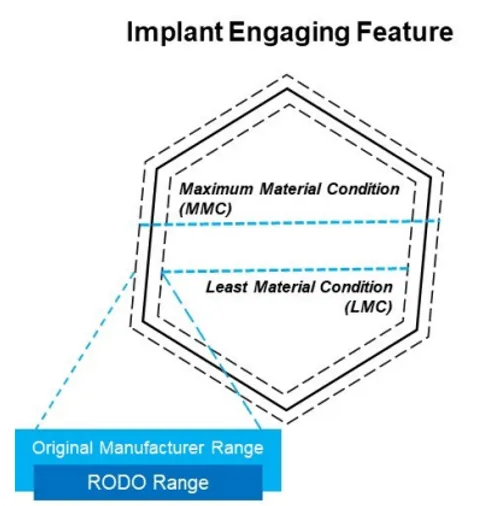
For the aforementioned reasons, RODO abutments have been designed with a range between the least material condition (LMC) and maximum material condition (MMC) that falls well within measurements taken during reverse engineering. This ensures that RODO components will always have equivalent or tighter tolerances than components from original equipment manufacturers they are meant to interface with (Figure 2). Importantly, these tolerances have undergone fatigue testing and have been validated by receiving FDA clearance.