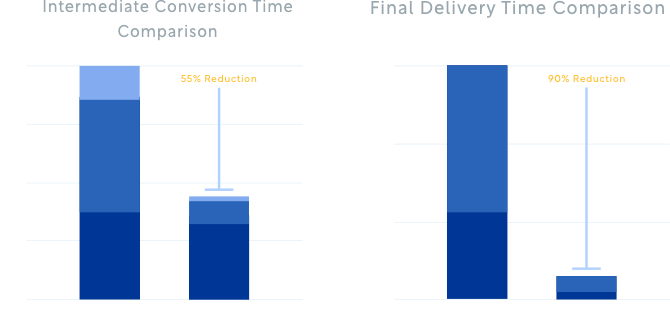
Q&A
Q: How does RODO restorative solution, Smileloc, differentiate itself from other conventional treatments; Screw-Retained or
Cement Retained dental prosthesis?
A: Smileloc replaces the need for screw-retained or cement-retained dental prosthesis thus removing the complications
associated with conventional treatment methods. Smileloc delivery is easy, quick and predictable. Additionally, the Smileloc
can easily be retrieved with Smilekey.
Q&A
Q: What is Smileloc?
A: Smileloc is made of nickel titanium aka Nitinol (special metal alloy used in medical industry for more than 40 years) and
acts a mechanical lock between abutment and prosthesis. Nitinol has unique shape-memory and super elastic properties.
Q&A
How does Smileloc work?
A: Smileloc has 8 arms - 4 inner arms that engage an abutment undercut and 4 outer arms that engage a coping undercut that the
restoration is affixed to.
Q&A
Q: Is Nitinol safe? What if my patient has nickel allergies
A: Nitinol is used in various medical applications such as endodontic files and heart stents. Smileloc has been
thoroughly studied and approved by FDA to ensure patient safety and product efficiency. One study performed by RODO showed
that a bare Smileloc leached less nickel than ingested as part of our daily diet.
Q&A
Q: Is Smileloc Single-Use?
A: Per FDA requirements, Smileloc is labeled as a single-use component. As are with other restorative components
such as prosthetic screws and healing abutments.
Q&A
Q: How is the Smileloc dental prosthesis removed?
A: Dental prosthesis can easily be removed with Smilekey, an induction device that emits energy to cause the 8
arms to taper and changes the Smileloc from a locked to an unlocked position enabling easy removal of the dental prosthesis.
Q&A
Q: How long does it take to “unlock” Smileloc with Smilekey?
A: Smilekey is preset to 8-seconds, for instance an all-on-four prothesis can be fully removed in less than a minute.
Q&A
Q: How long do I have to remove an arch before the Smileloc's arms re-engage?
A: Once the Smileloc is in the unlocked position, it will remain in that configuration. Only if the arms are
manually re-set would it return to a locked configuration.
Q&A
Q: At what temperature does the Smileloc unlock?
A: 55°C (135°F)
Q&A
Q: Will Smileloc unlock if my patient consumes very hot liquid or food?
A: No, direct heat needs to be applied to unlock Smileloc. In addition, Smileloc is insulated by the restorative
dental material (crown/prosthesis) and coping.
Q&A
Will Smilekey interfere with or cause damage to adjacent metal dental prostheses during activation? What about the implant,
will the heat damage to dental implant?
A: No, Smilekey activation is directed through the center of the tip and its magnetic fields dissipate into air
exponentially. The heat that is generated in Smileloc during activation for 8 seconds quickly dissipates and will not cause
damage to the implant.
Q&A
Q: What implant systems is Smileloc compatible with?
Q&A
Q: What gingival heights and angles are available for RODO Abutments?
A: RODO abutments are available in industry standard gingival heights and 17° and 30° for multi-unit application.
Q&A
Q: What torque value are RODO Abutments tightened to?
A: RODO abutments are tightened to the implant manufacturers' recommendations. The torque values are noted
on each product pouch. Note all RODO ancillary components should be hand tightened.
Q&A
Q: Does RODO offer custom abutments?
A: Custom abutment is not necessary with the RODO abutment system. Instead, you customize the tooth that will
connect to a stock abutment with the Smileloc. Since there is no residual cement clean-up required, a crown can be longer and
placed more subgingival and the emergence profile is not affected as it appears the tooth emerges from the gingiva for an
esthetic result.
Q&A
Q: What type of restorative material can be used with the RODO Abutment System?
A: RODO is agnostic to restorative material. Smileloc retained dental prostheses have been restored with Emax,
Zirconia, PEKKTON and TriLor to name a few.
Q&A
Q: Is the RODO Abutment System compatible with Dental Software Applications?
A: Yes, the RODO Abutment System library is available for 3Shape and EXOCAD.
Q&A
Q: What if my patient moves and the treating dentist is not knowledgeable about the RODO Abutment System?
A: RODO will loan any instruments and/or Smilekey required to the treating dentist. Additionally, RODO will
provide training and support to the treating dentist.
Q&A
Q: What is the longevity of the system?
A: RODO products come with a lifetime warranty on all components, except Smilekey. We completed FDA required
bench top testing exerting load and fatigue of more than 5 million cycles without failure in our products. We have cases more
than 7 years old which exhibit no degradation of our products.
Q&A
Q: How long has the product been on the market?
A: First usage of Smileloc was in 2013 as part of FDA clinical trial to obtain regulatory approval. FDA approved
Smileloc in late-2016 and Smilekey was approved in mid-2018.
Q&A
Q: Will implant warranty be voided by implant manufacturer when used with RODO Abutment?
A: If the implant company refuses to honor their warranty for the implant (exclusively due to the use of the
Products), and the Clinician both meets the “Eligibility" criteria, then RODO will reimburse the Clinician for the actual
and verifiable purchase cost of a replacement implant. Reference RODO Warranty Terms and Conditions for full coverage details
at
http://rodomedical.com/terms-and-conditions/.